Abstract
Background
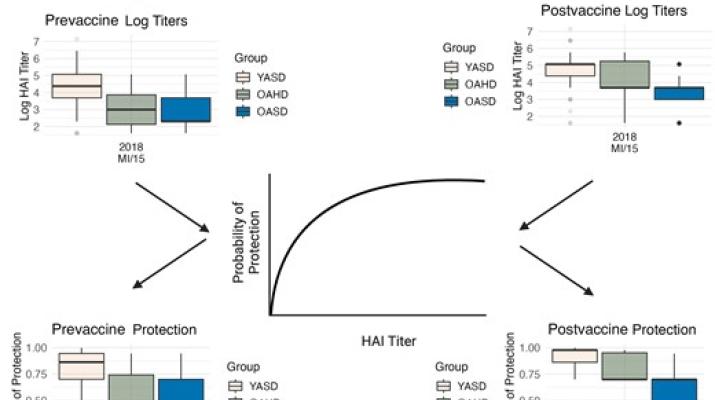
The high-dose (HD) Fluzone influenza vaccine is recommended for individuals aged ≥65 years due to its improved antibody responses and vaccine efficacy (VE) over the standard-dose (SD) formulation. Since influenza vaccines are frequently reformulated, monitoring VE changes is crucial. Traditional efficacy trials are costly and time-consuming, but immunogenicity studies using hemagglutination inhibition (HAI) titers—a reliable correlate of protection—can be used to estimate VE more efficiently.
Methods
We analyzed data from a human vaccine cohort who received either the SD or HD Fluzone split-inactivated influenza vaccine during influenza seasons 2013–2014 to 2021–2022. We used a previously developed statistical model to map pre- and postvaccination HAI titers to protection probabilities, and computed differences in VE of the HD vaccine in older (≥65 years) populations compared to SD vaccines in the same age group and in younger (<65 years) adults.
Results
We found that the HD vaccine generally improved the estimated VE in older adults. We also found that HD recipients often had a lower estimated VE than younger SD recipients.
Conclusions
While HD vaccines lead to a small increase in estimated VE compared to SD in older adults, further increases in dose or other developments to improve VE should be considered.